Recently, the Shanghai Municipal Science and Technology Commission announced the evaluation results of the Shanghai Professional Technical Service Platforms for 2023. Shanghai Biomaterials Testing Professional Technical Service Platform operated by the Shanghai Biomaterials Research and Testing Center was awarded the Excellent rating, receiving high praise from evaluation experts and relevant authorities. A total of 83 professional technical service platforms were involved in this evaluation, with 10 of them, including the aforementioned platform, achieving the Excellent result.
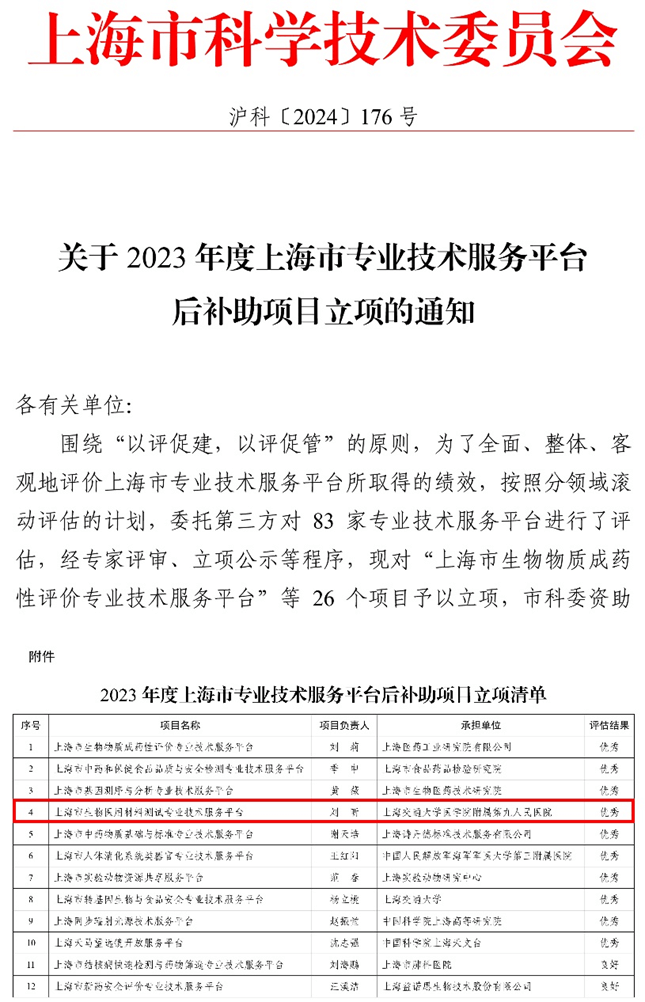
The Shanghai Biomaterials Testing Professional Technical Service Platform ( referred to as the Testing Platform), relying on the Department of Dental Materials (Shanghai Biomaterials Research and Testing Center) of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, is one of the first batch of professional technical service platforms in Shanghai. Led by Researcher Liu Xin as its director, the Testing Platform has obtained accreditation from the National Medical Products Administration (NMPA), China Metrology Accreditation (CMA), and China National Accreditation Service for Conformity Assessment (CNAS) as a testing and inspection institution, meeting the standards of ISO/IEC 17025, General Requirements for the Competence of Testing and Calibration Laboratories. In the field of biological evaluation of medical devices and biomaterials, it not only possesses testing capabilities that are synchronized with national or industry standards in China but also has advanced testing capabilities that are in line with international standards such as ISO, enabling it to issue legally binding medical device registration inspection reports and evaluation reports in both Chinese and English for domestic and international clients.
The Platform has long been committed to the research and development of novel dental and biomaterials, the exploration of material-body interactions and their mechanisms, the formulation and revision of national and industry standards, and the evaluation of the biological safety of materials. It has carried out a series of leading domestic and internationally synchronized research work, contributing to the transformation and application of domestic oral biomaterials and innovative medical devices.
This Excellent rating is a high affirmation of the Testing Platform's long-term efforts and a broad recognition of the research and testing staff's achievements in the R&D, innovation, and clinical translational safety evaluation of oral biomaterials and medical devices. On behalf of the Testing Platform, Director Liu Xin expressed gratitude to the hospital leadership and various functional departments for their long-term trust and support. He also stated that the Testing Platform would take this opportunity to continue providing public, open, and comprehensive professional technical services in line with the deployment and requirements of the Shanghai Municipal Science and Technology Commission, meeting the needs of technological innovation and economic and social development. It aims to continuously lead and promote the development and technological progress of the biomaterials/medical device evaluation industry in Shanghai, the Yangtze River Delta region, and nationwide, supporting the construction of our hospital as a research-oriented hospital and making positive contributions to Shanghai's scientific and technological innovation service system.